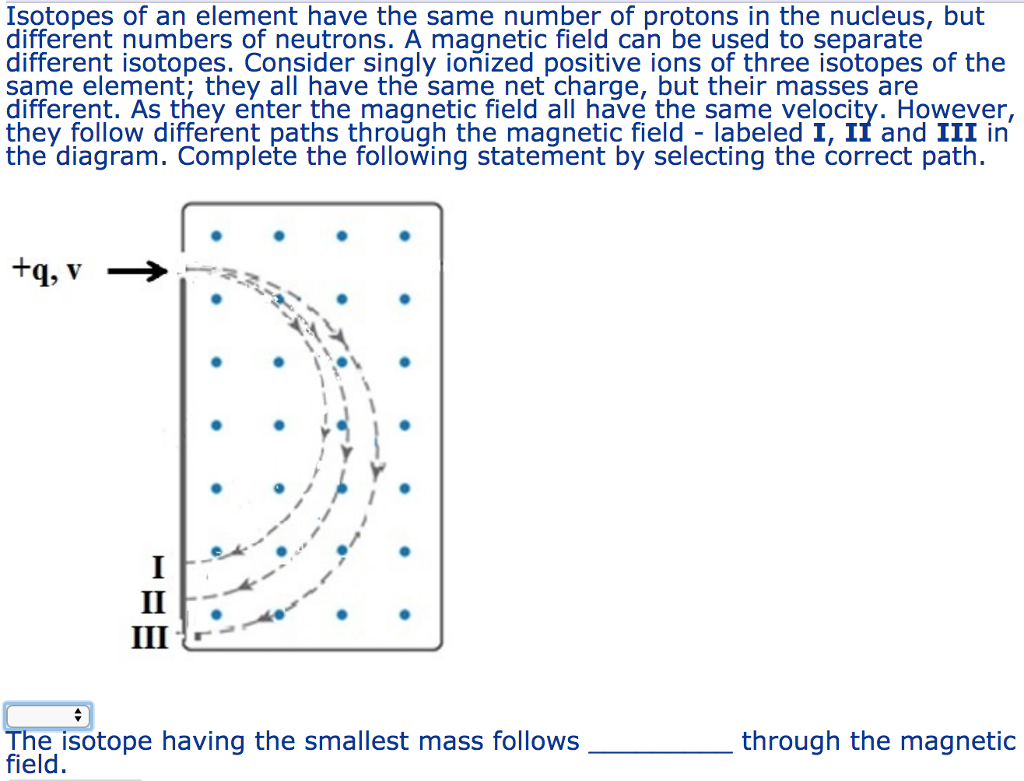
Each element has different isotopes. These isotopes have the same number of protons in their nucleus, similar chemical properties and the same element name. They differ only in the number of neutrons in their nuclei. Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25 Mg (10.13%), and 26 Mg (11.7%). The average atomic mass of the three isotopes is 24.3050 amu.
A family of people often consists of related but not identical individuals. Elements have families as well, known as isotopes. Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons.
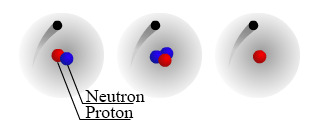
The number of protons in a nucleus determines the element’s atomic number on the Periodic Table. For example, carbon has six protons and is atomic number 6. Carbon occurs naturally in three isotopes: carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes.
The addition of even one neutron can dramatically change an isotope’s properties. Carbon-12 is stable, meaning it never undergoes radioactive decay. Carbon-14 is unstable and undergoes radioactive decay with a half-life of about 5,730 years (meaning that half of the material will be gone after 5,730 years). This decay means the amount of carbon-14 in an object serves as a clock, showing the object’s age in a process called “carbon dating.”
Isotopes have unique properties, and these properties make them useful in diagnostics and treatment applications. They are important in nuclear medicine, oil and gas exploration, basic research, and national security.

DOE Office of Science & Isotopes
Isotopes are needed for research, commerce, medical diagnostics and treatment, and national security. However, isotopes are not always available in sufficient quantities or at reasonable prices. The DOE Isotope Program addresses this need. The program produces and distributes radioactive and stable isotopes that are in short supply, including byproducts, surplus materials, and related isotope services. The program also maintains the infrastructure required to produce and supply priority isotope products and related services. Finally, it conducts research and development on new and improved isotope production and processing techniques.
Isotope Facts
- All elements have isotopes.
- There are two main types of isotopes: stable and unstable (radioactive).
- There are 254 known stable isotopes.
- All artificial (lab-made) isotopes are unstable and therefore radioactive; scientists call them radioisotopes.
- Some elements can only exist in an unstable form (for example, uranium).
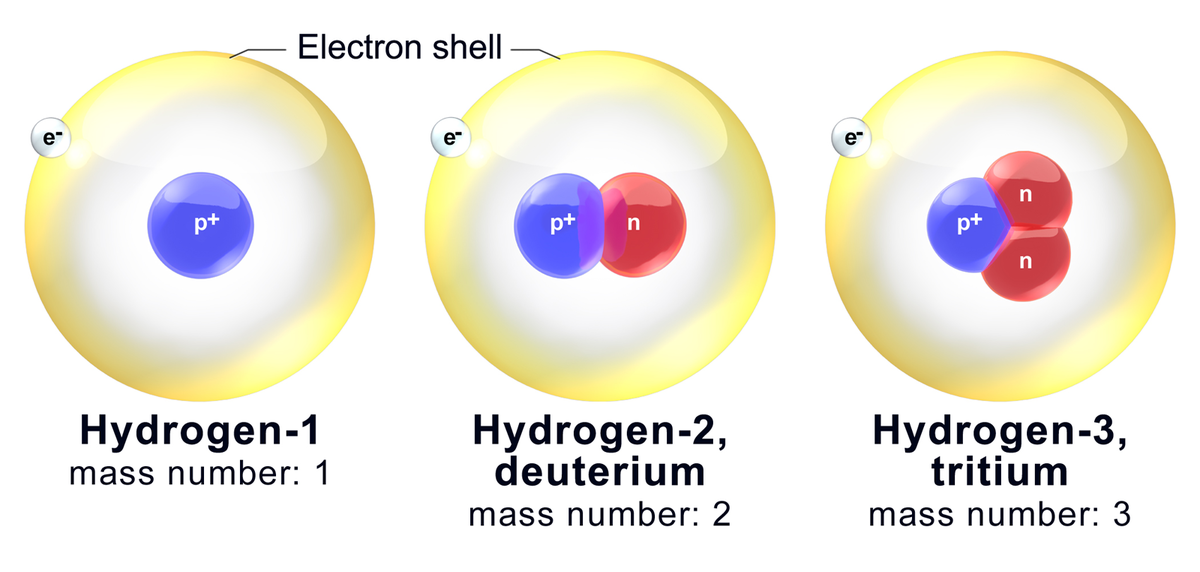
- Hydrogen is the only element whose isotopes have unique names: deuterium for hydrogen with one neutron and tritium for hydrogen with two neutrons.
Resources and Related Terms

- National Isotope Development Center (Isotope Basics)
Scientific terms can be confusing. DOE Explains offers straightforward explanations of key words and concepts in fundamental science. It also describes how these concepts apply to the work that the Department of Energy’s Office of Science conducts as it helps the United States excel in research across the scientific spectrum.
GENERAL CHEMISTRY TOPICS
Brave mac. The new Brave browser blocks ads and trackers that slow you down and invade your privacy. Discover a new way of thinking about how the web can work. At the top left, Open the Apple menu. Select “About This Mac”. In the “Overview” tab, look for.
Isotopes
An isotope is made up of atoms of the same element that have the same atomic mass. Different isotopes of an element arise from atoms with differing numbers of neutrons. Uses of isotopes. Average atomic masses from natural abundances: The weighted-average calculation.
Atomic number, mass number and isotopes
Musical accompaniment for these lecture notes.
The atomic number of an element (symbolized as Z) is the number of protons in the nuclei of its atoms. The mass number (A) is the total number of nucleons (neutrons and protons). An isotope is made up of atoms of the same element (which by definition have a characteristic and fixed atomic number) that also have the same mass number. Different isotopes of an element arise from atoms with differing numbers of neutrons. Because of this, chemists need a way to represent specific isotopes of an element. Isotopes of an element have the same atomic number, but different mass numbers. The atomic number, when represented along with the symbol of an element, is shown as a leading subscript. The mass number is shown as a leading superscript. Since the element symbol implies an atomic number, the latter is often dropped, and an isotope as commonly represented textually with just the mass number and the element symbol (for example 14C or 18O).
In the periodic table, the elements, represented as their symbols, are arranged in a particular pattern that reflects (as we will see) a regularity, or periodicity Malwarebytes for mac 10.6 8 free download. in their properties. Typically in the table, the element symbol is contained within its own small box, along with other information including the atomic number and the average atomic mass. The average atomic mass of an element represents the averages of its naturally occurring isotopic masses weighted according to their natural abundance. The formula for calculation of average atomic mass and illustration of its use is presented below.
How do the isotopic forms of an element differ from one another, physically and chemically? Isotopes are defined by their subatomic particle composition, which we will think of as a physical property. The chemistry of an element is determined by, in a general sense, the number of valence electrons its atoms possess. The number of valence electrons associated with a neutral atom is in turn determined by the number protons in the nucleus. Thus, two atomic nuclei could have the same number of protons, but different numbers of neutrons. Yet since the atoms they are part of would still have the same number of valence electrons, these two atoms would be chemically indistinguishable.*
Uses of isotopes
There are a wide variety of applications of isotopes in nuclear chemistry, medicine, biochemistry, anthropology, paleontology, and geology. Many such uses are based on the phenomenon of radioactivity, shown by some of the isotopes of many of the elements. Such radioactive isotopes are unstable, undergoing spontaneous nuclear decay processes at a rate determined by the half-life of the isotope. One example is the use of 14C - the isotope of carbon with six protons and eight neutrons, which has a half-life of 5730 years - as a basis for dating of materials derived from living organisms that are many thousands of years old. This technique, called radiocarbon dating, is used widely in geosciences and anthropology.
Average atomic masses from natural abundances: The weighted-average calculation
The atomic masses given in the periodic table represent weighted averages based on the natural abundances of the isotopes of a given element. The formula for a weighted average is
Here the xi's are the masses of the individual isotopes, and the wi's are the fractional abundances corresponding to the isotopes. Note that the weights must sum to 1 (equivalently the percent abundances must sum to 100%).
For example, chlorine exists in two isotopic forms, 35Cl and 37Cl. The mass of the 35Cl isotope is 34.97 amu and that of 37Cl is 36.97 amu. The abundances are 75.77% and 24.23%, repectively. Therefore in this case, the weighted average becomes
wa = (0.7577)(34.97 amu) + (0.2423)(36.97 amu) = 35.45 amu
The result of this calculation is the atomic mass of chlorine that appears in the periodic table.
Isotopes Of An Element Worksheet
* Actually, since isotopes of an element differ in atomic mass, they can be subtly distinguished by differences in reaction rates, or in physical processes - such as rate of diffusion - affected by mass.
